This article was originally published on the “Manure Scoop” by Dan Andersen on September 11, 2015 and has been modified and published here with his permission.
Every year we hear a chorus of reminders to wait until soil temperatures at the 4-inch depth are 50°F and trending cooler before applying anhydrous ammonia, and those of us in the manure world tend to echo these comments. That is if you are applying an ammonia rich manure, liquid/slurry hog manure wait until soils start to cool before applying. So, what is the science behind this recommendation, especially for manures?
This recommendation is based on the potential for nitrogen loss. Remember, there are a few forms of nitrogen that can be applied or are found in soils. These include ammonia/ammonium, nitrate, and organic nitrogen. Of these forms, all forms can be lost, but ammonia and nitrate tend to be the most mobile.
Ammonia is lost as a gas, so if we are using an ammonia/ammonium fertilizer (like swine manure) it’s important to get the fertilizer into the soil quickly where the ammonia will react with the soil particles and be held, rather than letting it sit on the surface where some of it can be lost to the air. This is why injection or immediate incorporation can be a great technique for getting the most from your manure, it makes sure that we aren’t immediately losing some portion of the nitrogen we are applying.
Nitrate on the other hand is lost with water, especially water moving through the soil to groundwater or tile drains. Nitrate is super soluble, so if water is moving and we have nitrate in our soil, it is probably moving with the water. We tend to have larger rains in the spring coupled with wetter soils from snowmelt; this means that if the nitrogen we applied is in the nitrate form there is a high opportunity for it to be lost in the spring.
When it comes to manures, it’s pretty much nitrate-free when we apply it, but microbes in the soil will process ammonium nitrogen and turn it to nitrate. The activity level of these microbes is controlled by how much ammonia is present, the amount of water and oxygen in the soil, and the soil temperature. Although all these variables are important, for now let’s focus just on temperature. A good rule of thumb is that microbial activity will double for every 18°F increase in temperature (so if our soil is at 68°F those microbes will be turning the ammonia in the manure into nitrate at about 2x the rate they would if our soil was at 50°F). Often times this means that not only will the microbes have more time to cause the conversion to nitrate, but they might be doing it much faster than if fertilizer application had waited.
So how do you know what the soil temperature is and whether it’s trending down? The Nebraska State Climate Office provides a daily report on the CropWatch website, and you can get more detailed local data at the Nebraska Mesonet website.
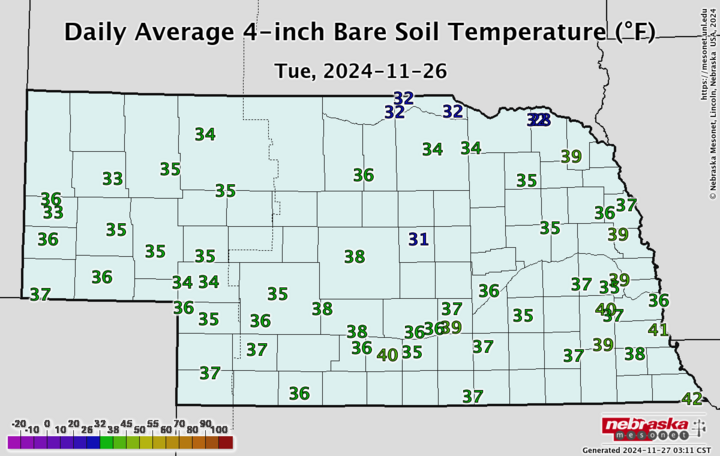
Nebraska State Climate Office).
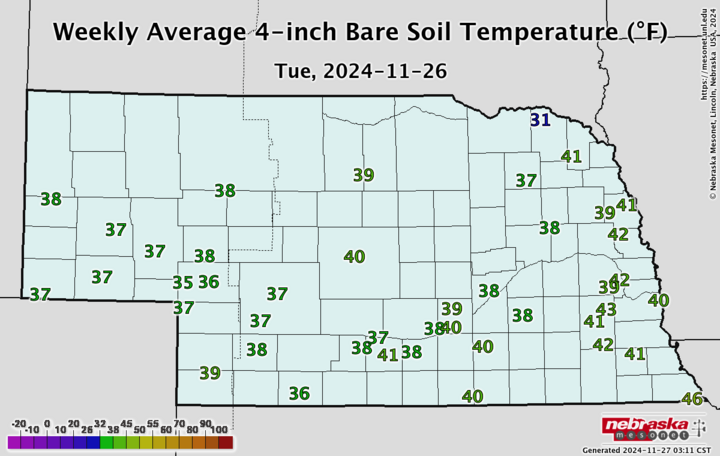
Nebraska State Climate Office).
Does nitrogen becoming nitrate mean we are going to lose it? No, it takes rainfall or snowmelt in the spring that will cause a leaching event, but it does increase the risk of loss. Recent heavy fall rains in some Nebraska locations may also be leaching soil nitrates from early fall manure applications .Certainly, there is a balance between making sure we get our manure applied before the soil freezes and applying too early, but hopefully the information above illustrates a bit behind the science of the 50°F and cooling recommendation.
This article was reviewed by Rick Koelsch
Interviews with the authors of BeefWatch newsletter articles become available throughout the month of publication and are accessible at https://go.unl.edu/podcast.
